

(Food intake may be considered work done on the body.) (b) Plants convert part of the radiant energy in sunlight into stored chemical energy, a process called photosynthesis.

Heat transferred out of the body (Q) and work done by the body (W) remove internal energy, whereas food intake replaces it. It can also be used to describe how energy transferred by heat is converted and transferred again by work.įigure 12.7 (a) The first law of thermodynamics applies to metabolism. The first law of thermodynamics applies the conservation of energy principle to systems where heat and work are the methods of transferring energy into and out of the systems. In order to understand the relationship between heat, work, and internal energy, we use the first law of thermodynamics. It can be divided into many subcategories, such as thermal and chemical energy, and depends only on the state of a system (that is, P, V, and T), not on how the energy enters or leaves the system. Internal energy is the sum of the kinetic and potential energies of a system’s atoms and molecules. However, both can change the internal energy, U, of a system. Heat and work are both energy in transit-neither is stored as such in a system.
Once the temperature increase has occurred, it is impossible to tell whether it was caused by heat or work. Similarly, work can be done on the system, as when the bicyclist pumps air into the tire. Heat transfers energy into a system, such as when the sun warms the air in a bicycle tire and increases the air’s temperature. For example, both can cause a temperature increase. Nevertheless, heat and work can produce identical results. Heat is driven by temperature differences, while work involves a force exerted through a distance.
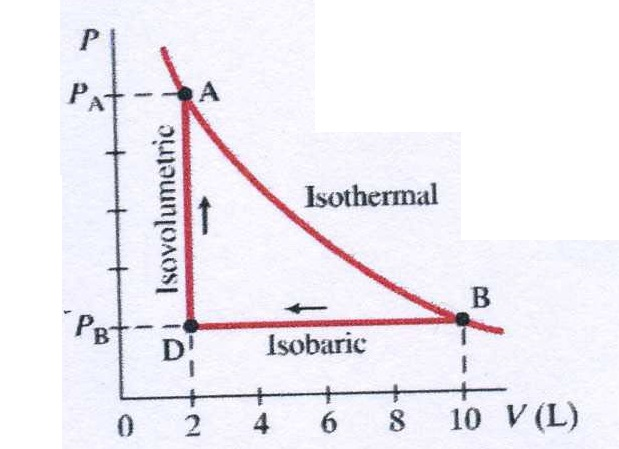
Heat ( Q) and work ( W) are the two ways to add or remove energy from a system. If you continue to pump air into tire (which now has a nearly constant volume), the pressure increases with increasing temperature (see Figure 12.4).
Calculating workdone with temperature increase full#
Once the tire has expanded to nearly its full size, the walls limit volume expansion. The tire’s volume first increases in direct proportion to the amount of air injected, without much increase in the tire pressure. To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into a deflated tire. This is why railroad tracks and bridges have expansion joints that allow them to freely expand and contract with temperature changes. Gases are especially affected by thermal expansion, although liquids expand to a lesser extent with similar increases in temperature, and even solids have minor expansions at higher temperatures. What is the underlying cause of thermal expansion? An increase in temperature means that there’s an increase in the kinetic energy of the individual atoms. This last point describes thermal expansion-the change in size or volume of a given mass with temperature. When pressure is constant, volume is directly proportional to temperature.When temperature is constant, pressure is inversely proportional to volume.When volume is constant, pressure is directly proportional to temperature.Instead, it is important for us to notice from the equation that the following are true for a given mass of gas: The constant k is called the Boltzmann constant and has the value k = 1.38 × 10 −23 J/K, k = 1.38 × 10 −23 J/K, For the purposes of this chapter, we will not go into calculations using the ideal gas law. Where P is the pressure of a gas, V is the volume it occupies, N is the number of particles (atoms or molecules) in the gas, and T is its absolute temperature.


 0 kommentar(er)
0 kommentar(er)
